Working directories
1 | i=/media/ht/ht_5T/Bioinformatics/Bioinfo-Softs/NCBI/Datasets |
Kaiju: Fast and sensitive taxonomic classification for metagenomics
Publication downloaded from URL as below
1 | i=/media/ht/ht_5T/Bioinformatics/Bioinfo-Softs/NGS_Softs/Kaiju |
Windows 10 VB download
1 | i=https://az792536.vo.msecnd.net/vms/VMBuild_20190311/VirtualBox/MSEdge/MSEdge.Win10.VirtualBox.zip |
Windows 10 Download Sites
- VMware 安装ghost win7 gho_yanchenyu365的博客-CSDN博客
- linux环境下使用virtualbox安装ghost系统_RunningStoic的博客-CSDN博客
- 轻松玩转VMWare虚拟机之安装GHOST系统图文教程_安装教程_脚本之家
- VMware虚拟机怎么安装ghost系统 - 简书
- ubuntu下用VirtualBox安装Windows虚拟机_Leefir的博客-CSDN博客_ubuntu windows虚拟机
- Win10竟然内置了一台虚拟机?教你如何玩转它-Windows 10,虚拟机 ——快科技(驱动之家旗下媒体)–科技改变未来
- 2020版Ghost Win10 64位下载 win10 gho - Win10专业版官网
- win10系统下载_win10正式版下载_ghost windows10免激活系统下载-系统城
- 系统之家win10系统安装盘_系统之家ghost windows10 64位纯净优化版v2020.12-系统城
- 微软发布Win10 20H2 Build 19042 ISO镜像下载 - 老王系统,www.win10gw.com
- Win10 2009正式版ISO镜像 - 老王系统
- Win10 2009下载_微软官网Win10 19042系统64位下载 - 老王系统,www.win10gw.com
- Win10 20h2正式版ISO镜像 - 老王系统
- Get the Windows 10 October 2020 Update
- What Is the Latest Version of Windows 10?
NSFC Application
1 | # |
自然科学基金个人信息。Gmail赵秀军国自然群中,最后一个文件。Word document created in RS Downloads directory.国自然交叉学科。截图下载- 2021年度国家自然科学基金委员会交叉科学部项目申请指南
- 信息科学部案例, P6
- Code, 医学, H2103, H1904. 肝炎病毒与感染
- Code, 生命科学, C06, 生物信息理论方法
Accessable directory in a mobile phone with command-line
1 | i=/run/user/1000/gvfs/ftp:host=192.168.1.8,port=2121,user=ht/Download/ |
Vaccine in US
App, v-safe

Priority and coverage of vaccine
- 5%
- 30%
- 40%
Immuno-suppresive
tacrolimus, cyclosporine, mycophenolate, azathioprine, sirolimus
- EpiPen 2-Pak, 过敏
Other names: Epinephrine, adrenaline, adrenalin
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration).. Adrenaline is normally produced both by the adrenal glands and by a small number of neurons in the medulla oblongata.
Generic Name: epinephrine injection (EP i NEF rin)
Brand Name: Adrenalin, Auvi-Q, Epinephrinesnap-EMS, Epinephrinesnap-V, EpiPen 2-Pak, EpiPen JR 2-Pak, EPIsnap, Symjepi
EpiPen 2-Pak is used to treat severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs, and other allergens.
Epinephrine auto-injectors may be kept on hand for self-injection by a person with a history of severe allergic reaction.
Epinephrine is also used to treat exercise-induced anaphylaxis, or to treat low blood pressure that is caused by septic shock.
EpiPen 2-Pak may also be used for purposes not listed in this medication guide.
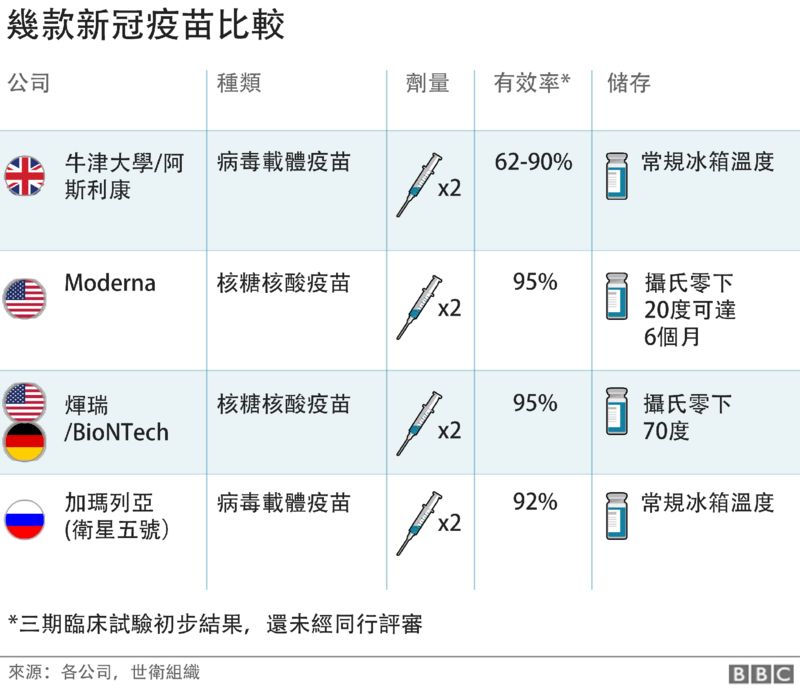
Pfizer
- Peter Doshi: Pfizer and Moderna’s “95% effective” vaccines—we need more details and the raw data - The BMJ
- Clarification: Pfizer and Moderna’s “95% effective” vaccines—we need more details and the raw data - The BMJ
- 44 K
- Placebo, 162, 9 heavy
- Vaccine, 8, 1 heavy
- 5 shots / vial, 0.3 ml / shot. Dilution needed
- Pfizer and BioNTech Announce Publication of Results from Landmark Phase 3 Trial of BNT162b2 COVID-19 Vaccine Candidate in The New England Journal of Medicine | Pfizer
- Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints | Business Wire
Moderna
- 30 K
- Placebo, 185, 30 heavy
- Vaccine, 11, 0 heavy
- 10 shots / vial, 0.5 ml / shot
The trial began on July 27, 2020, and enrolled 30,420 adult volunteers at clinical research sites across the United States. Volunteers were randomly assigned 1:1 to receive either two 100 microgram (mcg) doses of the investigational vaccine or two shots of saline placebo 28 days apart. The average age of volunteers is 51 years. Approximately 47% are female, 25% are 65 years or older and 17% are under the age of 65 with medical conditions placing them at higher risk for severe COVID-19. Approximately 79% of participants are white, 10% are Black or African American, 5% are Asian, 0.8% are American Indian or Alaska Native, 0.2% are Native Hawaiian or Other Pacific Islander, 2% are multiracial, and 21% (of any race) are Hispanic or Latino.
From the start of the trial through Nov. 25, 2020, investigators recorded 196 cases of symptomatic COVID-19 occurring among participants at least 14 days after they received their second shot. One hundred and eighty-five cases (30 of which were classified as severe COVID-19) occurred in the placebo group and 11 cases (0 of which were classified as severe COVID-19) occurred in the group receiving mRNA-1273. The incidence of symptomatic COVID-19 was 94.1% lower in those participants who received mRNA-1273 as compared to those receiving placebo.
Investigators observed 236 cases of symptomatic COVID-19 among participants at least 14 days after they received their first shot, with 225 cases in the placebo group and 11 cases in the group receiving mRNA-1273. The vaccine efficacy was 95.2% for this secondary analysis.
- 0.2-0.3 % death rate (Mortality rate) of flu
- 1.8 % death rate of COVID-19
Sputnik V
